Explain the Difference Between an Ion and an Isotope
Both have long half-lives. The number of protons must remain unchanged.
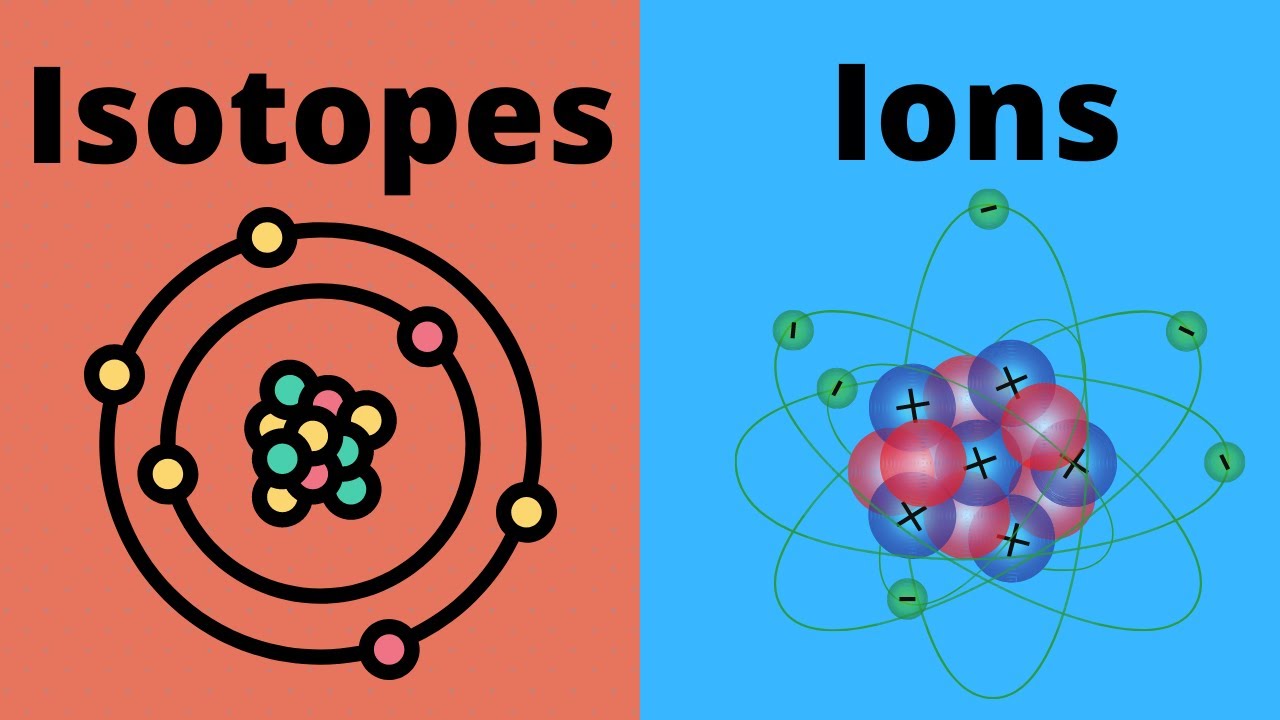
Isotopes Vs Ions What Is The Difference Youtube
Number of protons Atomic number Middle one or two letters first letter always capitalized Atomic symbol.

. Typically an atom has the same number of protons and neutrons. What is the difference between an atom ion and isotope. Since protons neutrons mass of the atom and when the number of neutrons change - mass of atoms too change hence ³⁵Cl has a mass of 35 and ³⁷Cl has a mass of 37.
What is an isotope. Atoms are made up of protons neutrons and electrons. An ion is an atom with an electrical charge positive or negativeAn isotope of chemical element is an atom having a different number of neutrons.
Protons Neutrons Atomic mass number Bottom left. 3Isotopes may be stable not automatically decaying or. What is the difference between hydrolysis and dehydration synthesis.
Analyze Both differ in the number of neutrons. Problem 1 Part A a Define the terms atom ion and isotope and explain the difference between C and SC b Calculate the radius of the electron orbital of the second excited state of a hydrogen atom. If there are more electrons than protons the species has a negative charge.
The type of atom depends on the number of protons. Explain the difference between an isotope and an ion. An isotope is an atom with a different number of neutrons than.
An ion is a non-neutrally charged atom. Explain the three types of bonding ionic covalent hydrogen with examples. Ions are atoms or molecules that have lost or gained electrons and have an electrical charge.
1Ions are positively or negatively charged atoms while isotopes are different variations of atoms in an element. An anion is a negative ion meaning a neutral atom has gained 1 or more electrons. Know the difference between the atomic number and the atomic mass weight.
Uranium-235 and uranium-238 occur naturally in the Earths crust. When the number of neutrons in an atom changes an isotope is formed. If the chemical species has more protons than electrons it carries a net positive charge.
As we can see atoms ions and isotopes are dependent on the number of one of the three subatomic particles. Take the ORIGINAL atomic number and subtract it by the isotopes mass How a isotope is set up. Isotopes are versions of a particular element that have different numbers of neutrons.
Carbon 12 and Carbon 14 are both isotopes of carbon one with 6 neutrons and one with 8 neutrons both with 6 protons. C A laser beam with a power of 1 nW has a wavelength of 658 nm. Cations are positively charged because they lack electrons which are negative.
O-16 C-14 H-1 Cl-37. Describe the role of water in hydrolysis and. They differ by having different number of neutrons.
Ions are charged species but isotopes are neutral. A few examples of ions are Na2 cation and F- anion A few examples of isotopes are Uranium-235 and Carbon-13. Cation Versus Anion Cations are ions with a net positive charge.
Isotopes of elements can participate in forming ions. This electrically charged atom is referred to as an ion. What is an ion.
The number of neutrons determines the isotope of an element but does not affect the electrical charge. These different atoms of the same element are called isotopes. And the ion depends on the electrons in an atom.
What is the difference between anion and isotope. Na Mg 2 Cl- O 2-. In ions the number of electrons is different from the number of protons.
An isotope is an atom of the same element that has different numbers of neutrons. 2Ions exist when there is a deficiency or excess of electrons in an atom while isotopes exist when there is a. What is the difference between an ion and isotope and a neutral atom.
Describe the difference between an ion and isotope. They are different from each other by having different numbers of neutrons. What are differences between ions and isotopes.
An Atom is the basic building parts of everything a ion is a molecule with a net electric charge and a isotope is energy ball of different number of protons and neutrons in the nuclei Advertisement kemanci kemanci. Since the neutron number is different their mass number also differs. An ion is an atom of the same element that has different numbers of electrons.
Isotopes of an atom have different atomic masses and exhibit different properties but they are still the same element. Carbon-12 is a stable isotope while carbon-14 is a radioactive isotope radioisotope. Whats the difference between an isotope and an ion of the same element.
But in a non-neutral atom there is a lack Cation or excess of electrons Anion -. Include the specific names for the two types of ions and one example of a use for isotopes in. Isotopes It has atoms that have the same number of protons but different number of neutrons.
Explain the difference between an. An isotope is an atom with a different number of neutrons zero charge to protons than the original atom. These combine to form the neutral ion of Sodium Chloride.
What is the main difference between an isotope and an ion. A neutral atom has the same amount of protons and electrons which is why it is neutral. Difference Between Isotope and Ion Isotopes are different atoms of the same element.
How many photons are emitted d The laser from c shines on a hydrogen atom in. The isotope of an element depends on the number of neutrons. The neutrons add to the weightmass of the atom but not to the overall charge.
Some atoms gain or lose a neutron. However the isotopes of the same element have the same number of protons and neutrons. Both of these are called isotopes Ions are atoms which have either gained an electron or lost an electron and are charged.
Solution for Explain the difference between an ion and an isotope. Change the number of neutrons in an atom and it becomes an isotope change the number of electrons it becomes an ion. Basically an ion is a charged particleatom.
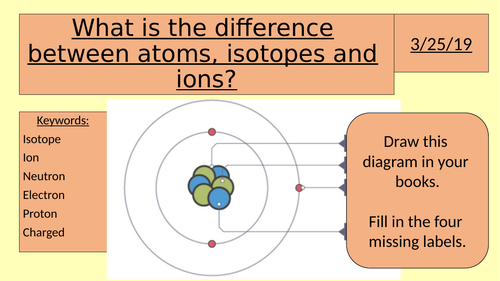
Difference Between Atoms Ions And Isotopes Teaching Resources

Isotopes Vs Ions The Difference Between Isotopes And Ions Youtube

No comments for "Explain the Difference Between an Ion and an Isotope"
Post a Comment